


Softek has manufactured under the highest quality of standards, systems, validation protocols and database interface tools.
Clients
Countries
Years
Supported


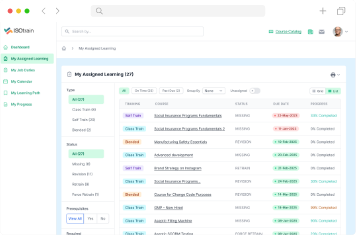
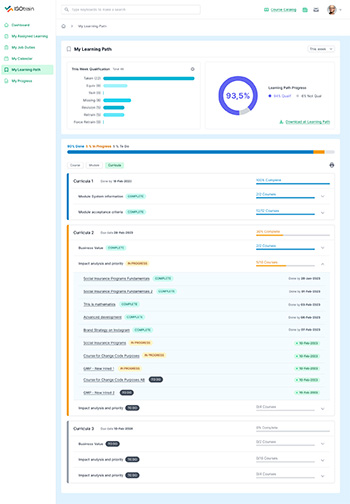
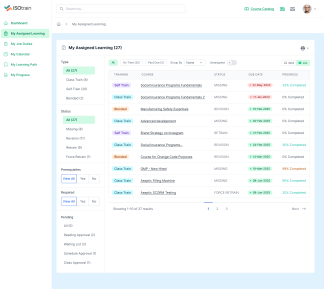
ISOtrain provides a range of features and benefits to help businesses optimize training initiatives and achieve goals.

Investing in career development is crucial for attracting and retaining top talent. ISOtrain LMS offers a comprehensive Career Development feature designed to empower employees and drive professional growth within your organization.

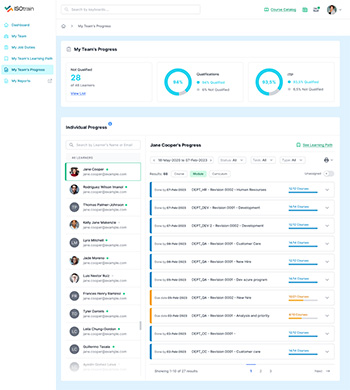
Maintaining compliance is essential for organizations across industries. ISOtrain LMS offers a robust Compliance Tracking and Reporting feature, designed to streamline compliance monitoring and facilitate detailed reporting for regulatory audits and inspections.

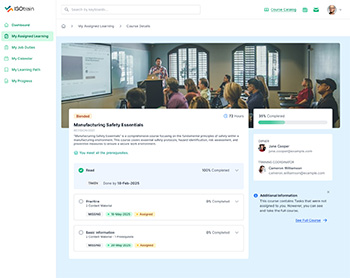
Empower your workforce with comprehensive on-the-job training capabilities offered by ISOtrain LMS. Our platform facilitates hands-on learning experiences directly within the workplace environment, ensuring that employees acquire the necessary skills and knowledge while performing their job duties.

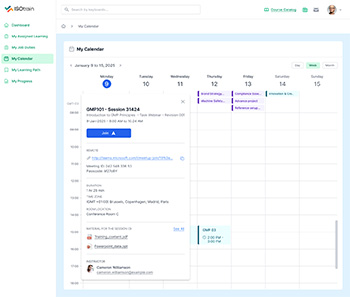
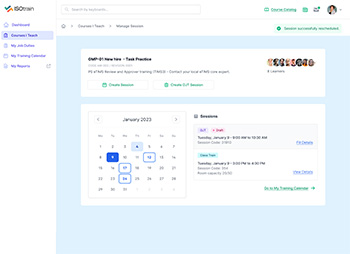
Stay on top of training schedules, compliance deadlines, and certification renewals with ISOtrain LMS’s automated notifications and reminders feature.

ISOtrain LMS offers a comprehensive Assessments feature designed to support your organization’s training and development needs.

Organizations face increasing pressure to maintain comprehensive training records while ensuring compliance with industry standards. Our E-Signature Integration in ISOtrain LMS offers a robust solution for electronically signing and managing training records and documents, enhancing efficiency and compliance.

Experience the ease of automated approvals with ISOtrain LMS. Our customizable pathways and role-based permissions make it simple for stakeholders to review and approve training requirements definition and training-related documents, reducing delays and ensuring compliance.

ISOtrain LMS prioritizes data security and provides a comprehensive audit trail to ensure the safety and integrity of your organization’s training records and sensitive information, meeting the FDA 21 CFR Part 11 requirements for electronic records.

ISOtrain LMS offers comprehensive support for SCORM (Sharable Content Object Reference Model) and xAPI (Experience API), enabling seamless integration with various e-learning content and tools.

ISOtrain LMS brings flexibility and accessibility to your training programs through its Mobile Learning feature.

ISOtrain LMS offers a robust Role-Based Access Control (RBAC) system designed to ensure that training data remains secure while providing relevant access to authorized personnel.

ISOtrain LMS offers a comprehensive integration capability, allowing you to connect with various systems and streamline your organization’s training and compliance processes.
Our Suite of Products helps companies worldwide meet corporate and regulatory agencies’ training requirements.
Compliance training is crucial for organizations to educate employees about laws, regulations, and company policies relevant to their roles. It mitigates risks, ensures ethical behavior, and maintains a culture of compliance within the organization.
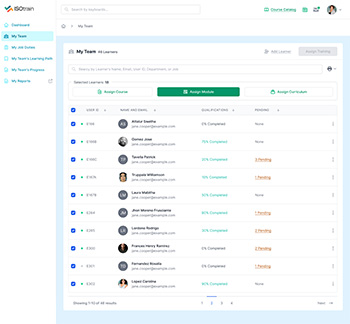
Employee onboarding is a critical process that sets the tone for new hires’ experiences and their success within the organization. With ISOtrain LMS, organizations can streamline and enhance their onboarding processes to ensure a seamless transition for new employees.
In today’s increasingly remote work environment, organizations need a robust Learning Management System (LMS) that can adapt to their workforce’s unique needs. ISOtrain LMS provides a comprehensive platform to support remote work, ensuring employees stay connected, trained, and compliant, regardless of location.
Employee development is crucial for maintaining a skilled and motivated workforce. ISOtrain LMS offers a comprehensive platform to support your organization’s employee development initiatives, providing the tools needed to build a culture of continuous learning, foster career growth, and improve overall performance.
Sales training is critical for building a successful sales team capable of driving revenue and meeting business goals. ISOtrain LMS provides a comprehensive platform to create, manage, and deliver effective sales training programs.
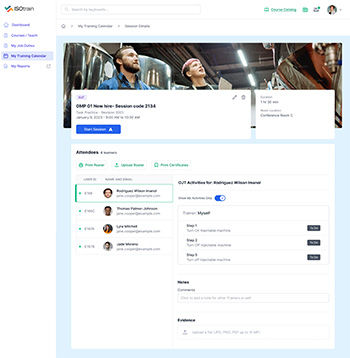
On-the-Job Training (OJT) is a vital approach for developing skills and competencies directly in the workplace environment. ISOtrain LMS provides a comprehensive platform that supports OJT, allowing employees to gain hands-on experience and practical knowledge while performing their job roles.
Small and medium businesses (SMBs) face unique challenges when it comes to training, compliance, and workforce development. ISOtrain LMS offers a comprehensive and scalable platform designed to meet the needs of SMBs, providing flexible training programs, seamless integration, and robust compliance tools.
Enterprise corporations require robust and scalable Learning Management Systems (LMS) to manage large-scale training programs, compliance requirements, and diverse workforce needs. ISOtrain LMS provides comprehensive solutions designed specifically for enterprise corporations, offering customizable training, detailed reporting, and seamless integration with existing systems.
ISOtrain is committed to assisting professionals in staying current on the most recent regulations and requirements. Our system is compliant with:
This FDA compliance regulation allows businesses to use electronic signatures and records instead of paper ones as long as your LMS has the necessary features. A 21 CFR Part 11-compliant LMS must allow regulatory auditors to track all data and operations through audit trails. FDA-compliant software should also use a secure hosting platform, have electronic training records and require electronic signatures before every significant operation.
ISOtrain also complies with the European Union (EU) General Data Protection Regulation (GDPR). The GDPR protects EU citizens’ privacy and personal information, and it applies to companies that operate within the EU or undertake business activities there. This regulation requires all businesses to state why they’re collecting a user’s personal information and how they intend to use it. The user portal functionalities in ISOtrain keep our LMS GDPR-compliant by allowing users to give their consent for data collection or specify their privacy preferences.
The European Medicines Agency (EMA) organizes inspections of businesses that develop, market, manufacture and sell medicine for human or veterinarian uses. ISOtrain ensures companies stay compliant by using specific features that allow EMA professionals to monitor and evaluate employee training history and other system operations.
Volume 4 of EudraLex outlines guidelines for good manufacturing practices (GMP) for human and veterinarian medicines. There are 19 Annexes within Volume 4, and Annex 11 refers explicitly to automated systems. Annex 11 states that a computerized system that replaces a manual one should be high-quality and low-risk. To comply with EudraLex, ISOtrain includes risk-management operations throughout the SDL. What’s more, we offer validation services with all products, and our systems feature built-in security checks with electronic signatures.
ISOtrain closely follows the Good Automated Manufacturing Practice (GAMP) guidelines from the International Society for Pharmaceutical Engineers (ISPE). The GAMP 5 provides practical instruction for how computerized systems in regulated industries can comply with current requirements while facilitating technological advancements. GAMP 5 also advises businesses on how they can improve the quality and efficiency of their computerized systems while decreasing overall costs.
With Compliance-Focused LMS Solutions, experience our cutting-edge tools and tailored approach, optimizing efficiency and effectiveness in regulated sectors.

